In the world of industrial processing and energy management, the
accurate measurement of gas flow is paramount. When specifying or operating a gas flow meter, you will encounter three common units of volumetric flow: m³/h, Nm³/h, and Sm³/h. While they may look similar, they represent fundamentally different concepts. Confusing them can lead to significant errors in process control, cost accounting, and safety compliance.
So, what is the difference, and why does it matter so much?
This guide will break down these units in the simplest possible terms, using a clear analogy to make the concept intuitive. By the end, you will understand exactly what each unit means and when to use it, ensuring you can select and operate your gas flow instruments with confidence.
The Fundamental Problem: Why Gas Volume is a “Moving Target”
Before we define the units, we must understand a basic principle of physics:
the volume of a gas is not a fixed property. It changes dramatically with changes in temperature and pressure.
Imagine you have a simple party balloon.
-
If you take the balloon from a warm room into the cold outdoors, it will shrink.
-
If you take that cold balloon back into a warm room, it will expand.
-
If you squeeze the balloon (increase its pressure), its volume will decrease.
The amount of air—the number of air molecules (i.e., the mass)—inside the balloon never changed. However, its volume was a constantly moving target depending on its environment.
Gas flowing through an industrial pipe behaves in exactly the same way. It is often hot and under pressure. To simply state its volume without referencing its temperature and pressure is an incomplete and often misleading piece of information. This is the problem that standardized units were created to solve.
A Clear Definition of Each Unit
Let’s use our balloon analogy to understand each of the three common gas flow units.
m³/h (Actual Cubic Meters per Hour) – “What You See”
-
Definition: m³/h, often written as Am³/h (where "A" stands for "Actual"), represents the actual volume of gas passing through the meter at the live process conditions. It is a direct measurement of the gas volume inside the pipe at that very moment, under that specific operating temperature and pressure.
-
Balloon Analogy: m³/h is like measuring the size of the balloon right now, inside the hot, pressurized factory where it is being used. It is the real, physical volume the gas is occupying at that instant.
-
Significance and Limitations: This is a "what you see is what you get" measurement. While it accurately describes the volume in the pipe, it is not useful for comparing gas quantities. For example, 100 m³/h of compressed air at 7 bar pressure contains eight times more air molecules (mass) than 100 m³/h of air at atmospheric pressure. Therefore, using m³/h for billing or efficiency calculations is unreliable without also stating the live temperature and pressure.
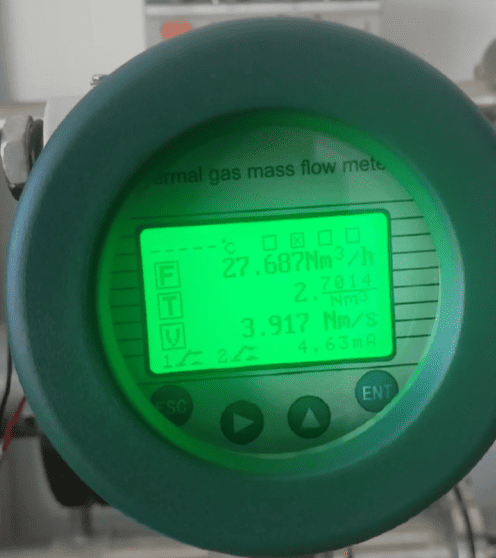
Nm³/h (Normal Cubic Meters per Hour) – “The European Standard”
Flow meter with Nm3/h
-
-
Definition: Nm³/h stands for Normal Cubic Meter per Hour. This is not a measurement of the actual volume in the pipe; it is a standardized measurement of the quantity (mass) of the gas. It answers the question: "If I took the gas flowing through my pipe and brought it to a set of universally agreed-upon 'Normal' conditions, what volume would it occupy?"
-
These "Normal Conditions" are internationally defined by standards like DIN 1343 and ISO 2533 as:
oTemperature: 20°C
oPressure: 1.01325 bar absolute (1 atmosphere)
-
Balloon Analogy: Nm³/h is like taking the hot, pressurized balloon from the factory floor, putting it into a special reference room that is at 20°C and standard sea-level pressure, and then measuring its size. No matter what its size was in the factory, if it contains the same amount of air molecules, its size in this cold, standard room will always be the same.
-
Significance and Value: Nm³/h is a measure of the mass of the gas, expressed as a volume. Because it uses a fixed, universal baseline, it allows for fair and accurate comparisons. It is the standard unit for most scientific and industrial gas measurement in Europe and many other parts of the world.
Sm³/h (Standard Cubic Meters per Hour) – “The American & Industrial Standard”
-
Definition: Sm³/h stands for Standard Cubic Meter per Hour. Conceptually, it is exactly the same as Nm³/h—it is a standardized measure of the quantity (mass) of the gas.
-
The Only Difference: The reference conditions used for "Standard" are different from "Normal." Unfortunately, there is no single universal definition for "Standard Conditions," but one of the most common, particularly in the United States and the oil and gas industry, is:
oTemperature: 15.6°C (60°F) or sometimes 15°C
oPressure: 1.01325 bar absolute (1 atmosphere)
-
Balloon Analogy: Sm³/h is like taking your balloon to a different standard room, this one set to a more common ambient temperature like 15.6°C. Because this room is warmer than the 0°C "Normal" room, the same balloon (with the same mass of air) will expand slightly. Therefore, a quantity of gas measured as 100 Sm³/h is the same mass as that same gas measured as approximately 94.5 Nm³/h.
-
Significance and Value: Like Nm³/h, Sm³/h is a reliable unit for billing, process control, and efficiency calculations. The critical takeaway is to always know which temperature and pressure base is being used when you see the term "Standard."
How Flow Meters Handle These Units
Understanding these units is key to choosing the right flow meter technology.
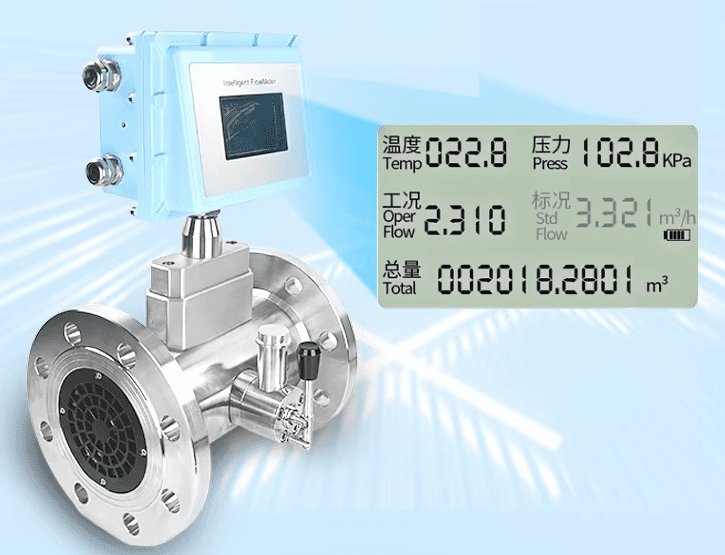
Gas turbine flow meter with built-in temperature and pressure sensor and flow computer to calculate Nm3/h
-
Volumetric Meters (e.g., Vortex, Turbine, Oval Gear, Rotameter): These instruments inherently measure the actual velocity or volume of the gas as it passes through, which is the m³/h reading. To provide a useful, standardized reading (Nm³/h or Sm³/h), they must be paired with separate pressure and temperature transmitters and a flow computer. The flow computer takes the live data from all three instruments and performs a continuous calculation to convert the actual volume to the standard volume. Gas turbine and vortex flow meters from silverinstruments.com feature built-in temperature and pressure sensors, along with integrated software for real-time calculation of standardized flow rate. A single meter provides multiple parameters: working flow, standardized flow, temperature, and pressure.
Vortex flow meter with steam mass flow measurement
-
Mass Flow Meters (e.g., Thermal Mass, Coriolis): These instruments are the direct solution to the problem. They measure the mass flow rate of the gas (e.g., in kg/h) directly. Since Nm³/h and Sm³/h are also representations of mass, these meters can use their built-in microprocessors to accurately and instantly convert the mass reading into the desired standardized volumetric unit (Nm³/h or Sm³/h) without the need for external compensation. Vortex flow meters are commonly equipped with the capability to determine steam mass flow by measuring temperature and pressure with an integrated calculator.
Quick Comparison Table
|
Unit
|
Full Name
|
Reference Conditions
|
What it Measures
|
|
m³/h
|
Actual Cubic Meter per Hour
|
The live process temperature & pressure
|
The "hot/pressurized" volume in the pipe
|
|
Nm³/h
|
Normal Cubic Meter per Hour
|
0°C & 1 atm
|
Standardized quantity/mass (European std.)
|
|
Sm³/h
|
Standard Cubic Meter per Hour
|
15.6°C (60°F) & 1 atm (Common US std.)
|
Standardized quantity/mass (American std.)
|
Understanding the difference between actual and standard gas flow units is fundamental for any engineer or technician. In short:
-
m³/h tells you what the gas volume looks like inside your pipe right now.
-
Nm³/h and Sm³/h tell you how much gas you actually have, providing a stable basis for comparison and calculation.
For any application involving billing, combustion control, or efficiency reporting, always rely on standardized units. By understanding these concepts, you can better select the right gas flow meter and ensure your measurements are always accurate, comparable, and meaningful.
How to Convert m³/h to Nm³/h or Sm³/h
Gas is compressible, so its volume depends strongly on temperature and pressure. This means that the same quantity of gas may have very different volumetric flow readings if measured at different conditions. That is why engineers often distinguish between actual cubic meters per hour (m³/h) and normalized or standard cubic meters per hour (Nm³/h or Sm³/h).
The Conversion Formula
The general formula to convert actual gas flow to standard conditions is based on the ideal gas law:
Where:
-
QN = flow rate at standard or normalized conditions (Nm³/h or Sm³/h)
-
QA = actual flow rate at operating conditions (m³/h)
-
PA = absolute pressure at operating condition (bar or kPa)
-
PN = absolute pressure at standard condition (e.g., 1.01325 bar)
-
TA = absolute temperature at operating condition (Kelvin)
-
TN = absolute temperature at standard condition (e.g., 273.15 K for 0 °C)
For example, if gas flows at 50 m³/h at 3 bar(g) and 40 °C, you would first convert 3 bar(g) to absolute pressure (4 bar abs), then apply the formula to calculate Nm³/h.
A Simplified Approximation
In many day-to-day industrial cases where precise accuracy is not critical, technicians often apply a rough shortcut:
This method ignores temperature but provides a quick estimate. For example, 10 m³/h measured at 5 bar(g) could be roughly estimated as 50 Nm³/h.
Quick Reference Conversion Table
|
Actual Flow m³/h
|
Pressure (bar)
|
Approximate Nm³/h (m³/h × Pressure)
|
|
5
|
1
|
5
|
|
10
|
2
|
20
|
|
15
|
3
|
45
|
|
20
|
4
|
80
|
|
25
|
5
|
125
|
This table is a simple and practical guide for quick calculations, suitable for rough checks but not for billing or custody transfer.


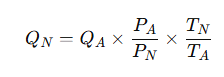
 SCFM air flow meter2019/07/14Thermal mass flow meter with digital display to show air instant flow and air total flow:Instant flow units available :SCFM,g/min,g/s,Kg/min,Kg/h,Nm3/h,Nm3/min,NL/h,NL/min,Total flow units available:CFM,g...VIEW
SCFM air flow meter2019/07/14Thermal mass flow meter with digital display to show air instant flow and air total flow:Instant flow units available :SCFM,g/min,g/s,Kg/min,Kg/h,Nm3/h,Nm3/min,NL/h,NL/min,Total flow units available:CFM,g...VIEW Gas Turbine Flow Meter with Temp & Press. compensation2018/08/27SGW-D series Gas turbine flow meter can be used to measure clean air or gas, such as natural gas, nitrogen gas.,etc. The best part of SGW series is that it can have integral temperature and pressure c...VIEW
Gas Turbine Flow Meter with Temp & Press. compensation2018/08/27SGW-D series Gas turbine flow meter can be used to measure clean air or gas, such as natural gas, nitrogen gas.,etc. The best part of SGW series is that it can have integral temperature and pressure c...VIEW Flanged Vortex flow meter with compensation2019/07/02STLU-BPT Series Flanged Vortex flow meter with integrated temperature sensor and pressure sensor compensation is a perfect choice for gas or steam (saturated steam and overheated steam) flow measureme...VIEW
Flanged Vortex flow meter with compensation2019/07/02STLU-BPT Series Flanged Vortex flow meter with integrated temperature sensor and pressure sensor compensation is a perfect choice for gas or steam (saturated steam and overheated steam) flow measureme...VIEW Thermal Mass Flow Meter2017/05/27Low cost gas flow meter.
Thermal Mass Flow Meter2017/05/27Low cost gas flow meter. Gas flow meter ml/min2023/07/14We supply ultra low flow gas flow meter can detect air, Hydrogen,mix gas, biogas, CO2, N2, oxygen gas at very small flow rate even with around 2 ml/min.VIEW
Gas flow meter ml/min2023/07/14We supply ultra low flow gas flow meter can detect air, Hydrogen,mix gas, biogas, CO2, N2, oxygen gas at very small flow rate even with around 2 ml/min.VIEW Metal Tube Rotameter2017/04/12HH5 Variable Area flow meter is Metal Tube Rotameter, get price now for digital rotameter,flow indicator rotameter.VIEW
Metal Tube Rotameter2017/04/12HH5 Variable Area flow meter is Metal Tube Rotameter, get price now for digital rotameter,flow indicator rotameter.VIEW